Pfizer COVID vaccine
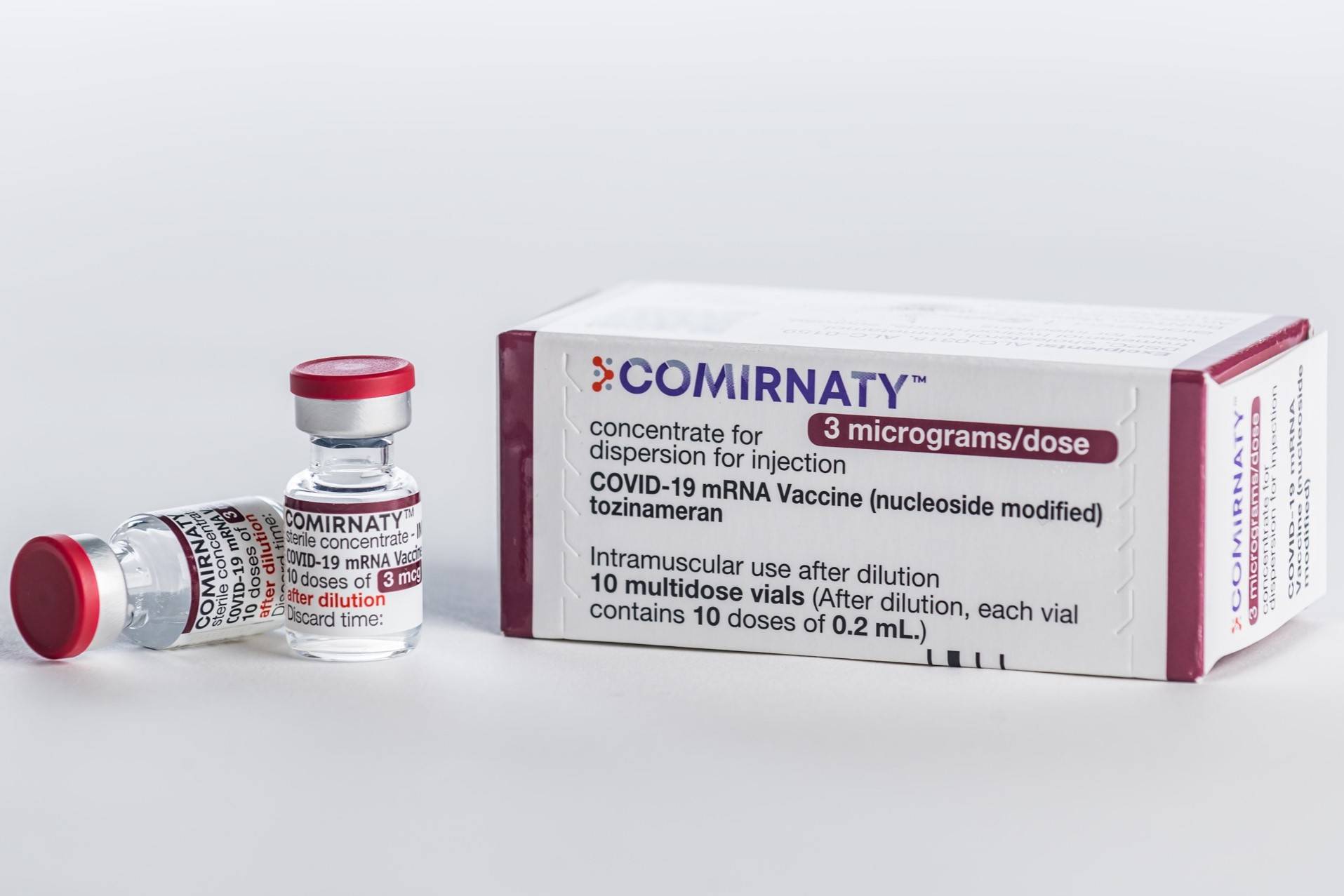
Pfizer- BioNTech COVID-19 Vaccine administer the second dose at least 21 days 3 weeks after the first dose. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine and the approved vaccine is marketed as Comirnaty for the prevention of COVID-19 in individuals 12 years of age and older.
Pfizer Covid Vaccine Appears 73 Effective In Children Under 5 Whyy
The Therapeutic Goods Administration TGA has provisionally approved the Comirnaty Pfizer vaccine for children aged 5 years and over.
. Prior revisions in 2021 129 2022 17 211 222 329 520 624 Page 1 of 4 Emergency Use Instructions EUI Fact Sheet for Recipients and Caregivers. Pfizer profits massively from repeated COVID-19 treatments. What Healthcare Professionals Need to Know Credit expires 12162022 Due to the ever-changing dynamics of COVID vaccine content is subject to change.
The vaccine manufacturer Moderna sued Pfizer and BioNTech on Friday claiming that its rivals Covid-19 shot copied groundbreaking technology that Moderna had developed years before the pandemic. Overall most adverse event reports were from females 726. For countries where the relevant regulatory authority has not authorised the vaccine or has not authorised it for an age group listed below the vaccine is investigational and its safety and efficacy have not been established.
Pfizer is the second vaccine to be approved for the under-fives At the moment only at-risk children in the age group are eligible for a COVID vaccine The paediatric dose contains a lower amount of. Find out about how to stay up to date with COVID-19 vaccines. Pfizer-BioNTech COVID-19 Vaccine Storage and Handling SummaryrRefer to CDC s Vaccine Storage and Handling Toolkit for best practices for vaccine storage and handlingr1.
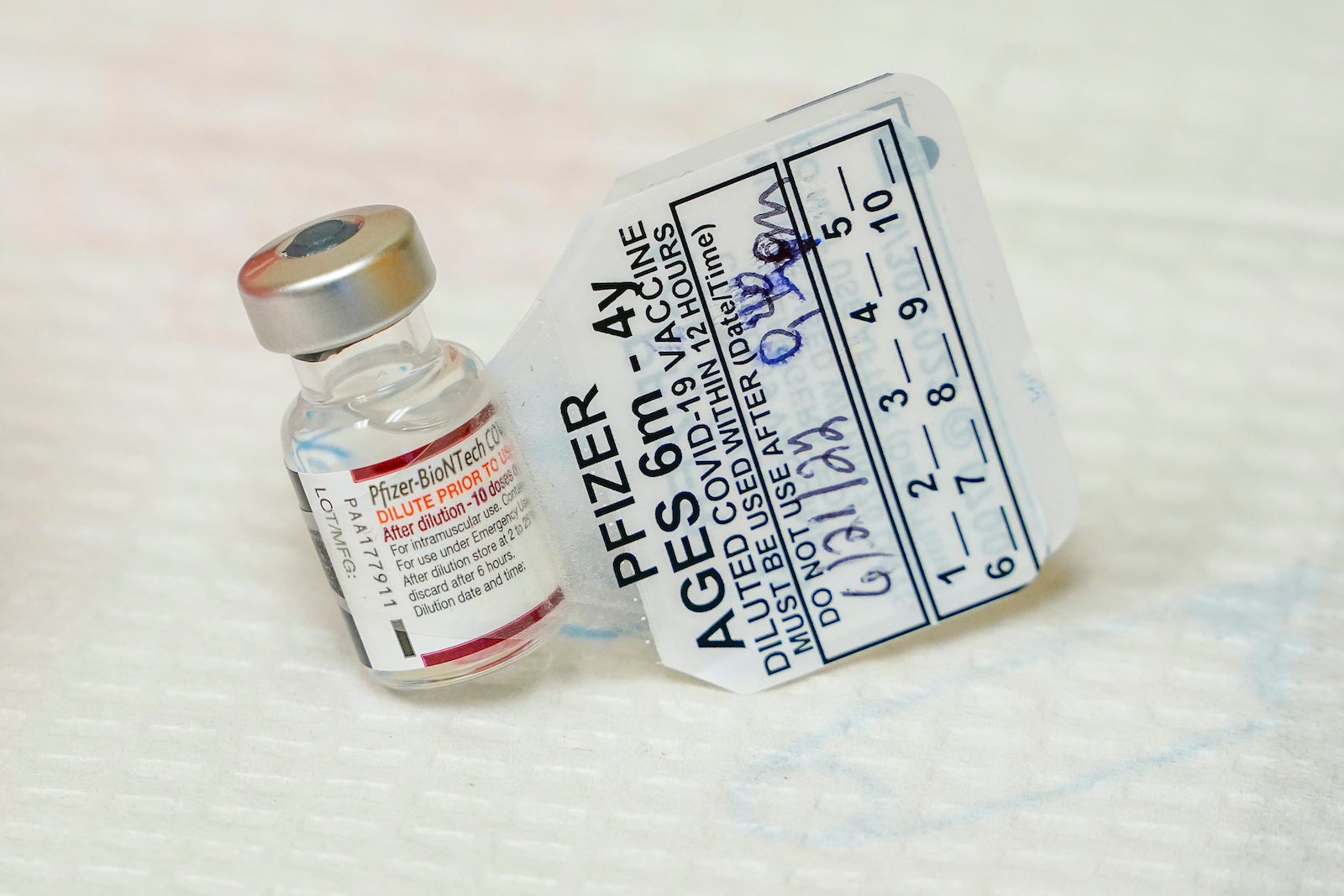
In authorising the use of Pfizers Comirnaty Covid-19 vaccine for children aged six months through four years HSA said it carefully considered the clinical data and assessed that the benefits outweighed the risks. COVID-19 Moderna Vaccine EUI Fact Sheet for Recipients. There are 24 adverse event reports in 11 year olds who likely received the Pfizer-BioNtech Comirnaty COVID-19 vaccine dose for individuals 12 years of age and older.
Completed Vaccine Administration Form. The approval status of the PfizerBioNTech COVID19 Vaccine varies worldwide. Pfizer and BioNTechs two-dose Covid vaccine provided very little protection for children aged 5 to 11 during the wave of omicron infection in New York according to a study published Monday.
11 2020 the Pfizer-BioNTech COVID-19 Vaccine has been available under EUA for individuals 16 years of age and older. COVID-19 Pfizer Vaccine EUI Fact Sheet for Recipients. Please bring the following to your visit.
From December 2020 to December 2021 about 470 million doses of. If the first-dose vaccine product cannot be determined or is. All three companies COVID-19 vaccines used mRNA.
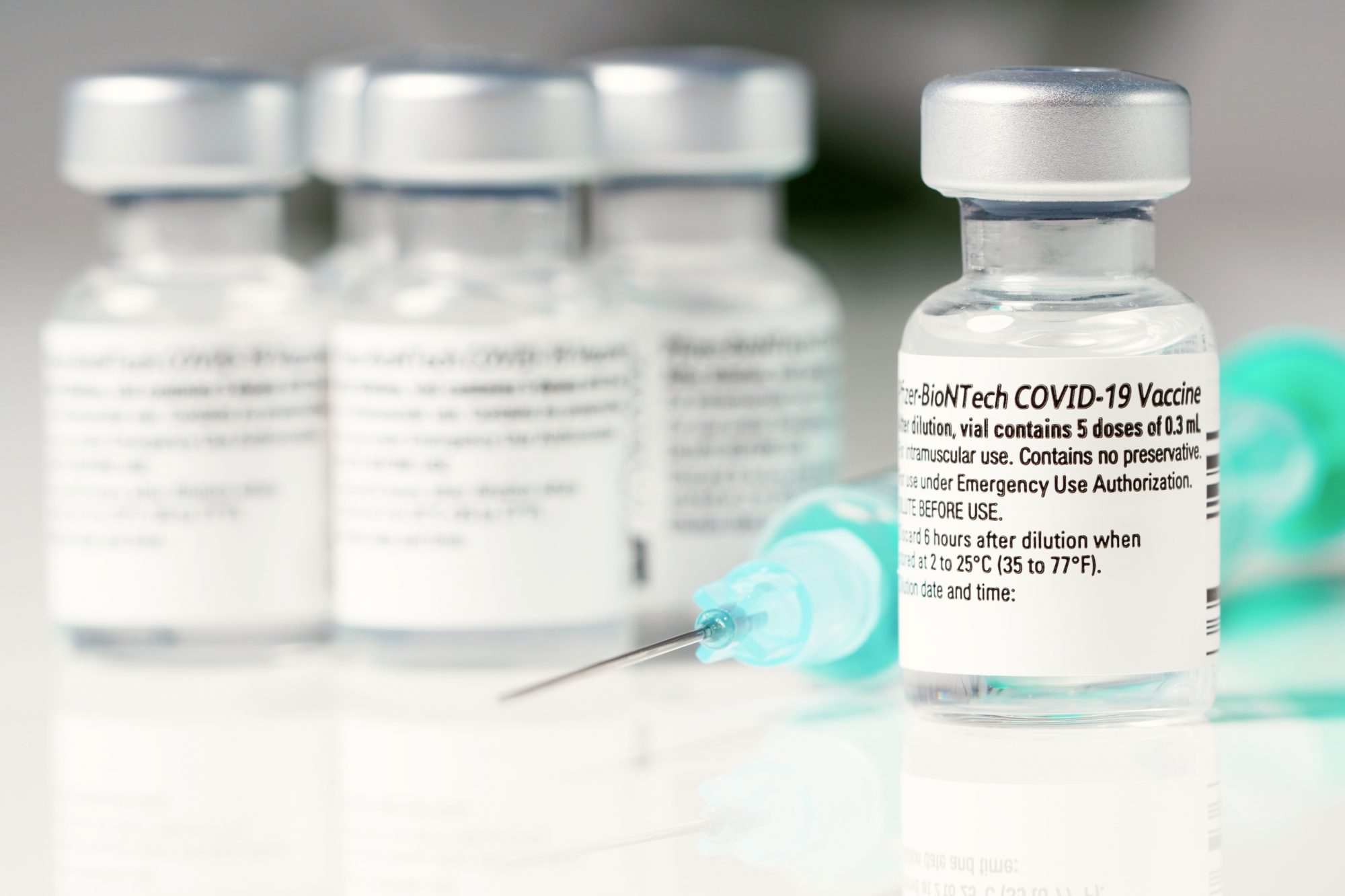
Pfizer-BioNTech COMIRNATY COVID-19 Vaccine. Each vial of the vaccine contains 5 doses of 03 milliliters. If the recipient has received 1 previous dose of.
Pfizer vaccine for 5 to 11 year olds. The results said HSA showed that the immune response in young children with a three-dose primary series was comparable to. With a current total of 556106 deaths.
The vaccine must be. The Pfizer and Moderna vaccines are strongly recommended as safe and effective at preventing serious illness or death from COVID-19. COVID-19 Novavax Vaccine EUA Fact Sheet for Recipients.
On 29 September 2022 the Therapeutic Goods Administration TGA provisionally approved a paediatric dose of Pfizers COVID-19 vaccine COMIRNATY tozinameran for children aged 6 months to less than 5 years. In an effort to eliminate more severe outcomes of the virus such as death and hospitalization the vaccine plays an important role in safety and prevention. Use purpose-built or pharmaceutical-grade units designed to either refrigerate or fr eezer2.
Pfizer-BioNTech COVID-19 Vaccine for Primary andor Additional Doses. What is the recommended dosage. Every vaccine storage unit must have a digital data logger temperature.
The Omicron BA4BA5-adapted bivalent vaccine contains 15- microgram of mRNA encoding the wild-type spike protein of SARS-CoV-2 in the Original Pfizer-BioNTech COVID-19 Vaccine and 15- microgram. Countries that have not authorised. Data show that following up Johnson Johnsons vaccine with a booster of an mRNA vaccine creates a stronger immune response and lessens the risk of serious side effects the Centers for Disease.
The authorization was expanded on May 10 2021 to include those 12. Please refer back to the. In the above episode of The Jimmy Dore Show Dore explains how the National Institute of Allergy and Infectious Diseases NIAID an arm of the National Institutes of Health has funded controversial gain-of-function research on bat coronaviruses at the Wuhan Institute of Virology which many believe.
Most kids 5 and older are currently eligible for a single booster dose of Pfizers COVID-19 vaccine at least five months after their second primary dose. For all persons aged 12 years and above SAGE recommends two doses 30 µg 03 ml each 4-8 weeks apart given intramuscularly into the deltoid muscle. Vaccinating children can help protect children from getting COVID-19.
11 Things You Need to Know. Pfizer-BioNTech COVID-19 Vaccine EUI Recipient Fact Sheet ver 9022022. According to the CDCs daily data tracker updated every evening at 8pm ET as of April 8 2021 there have been 30737477 cases of COVID-19 reported in the US.
As seen with older age groups it is expected that vaccines for younger children will provide protection from the most severe COVID-19. These reports are included in the 5 to 11 year age group. Insurance card including Medicare part B Pharmacy or Medical Coverage cards.
Nate Silver claimed liberal public health elites pressured Pfizer to delay fast-track approval of its COVID-19 vaccine until after the 2020 presidential election thus denying then. It argues that Pfizer and BioNtechs vaccine infringes patents Moderna filed between 2010 and 2016 for its messenger RNA or mRNA technology. If the recipient has never received a COVID-19 vaccine administer 1 dose of Pfizer-BioNTech COVID-19 Vaccine.
For more about the vaccine see Pfizers Covid Vaccine. The Pfizer BioNTech vaccine against COVID-19 has very high efficacy against severe disease and moderate efficacy against symptomatic SARS-CoV-2 infection. COVID-19 Vaccine Safety What We Know.
Immunocompromised kids are eligible for.
Japan Approves Pfizer Covid Vaccine For Use With Young Children The Japan Times

Norway Raises Concern About Pfizer S Covid 19 Vaccine Safety

Pfizer Biontech Covid 19 Vaccine Wikipedia
Ri1gukekfhcysm
Pfizer Did Not Know Whether Covid Vaccine Stopped Transmission Before Rollout

Pfizer Coronavirus Vaccine Corona Virus Covid 19 Covid Vaccines Panoramic Photo Stock Foto Adobe Stock
Pfizer And Moderna Raise Prices For Covid 19 Vaccines In Eu Ft Reuters
Pfizer Vaccine Vials Hold Some Extra Doses Experts Say That S Normal

Fda Documents Show Pfizer Covid Vaccine Protects After 1 Dose Cidrap
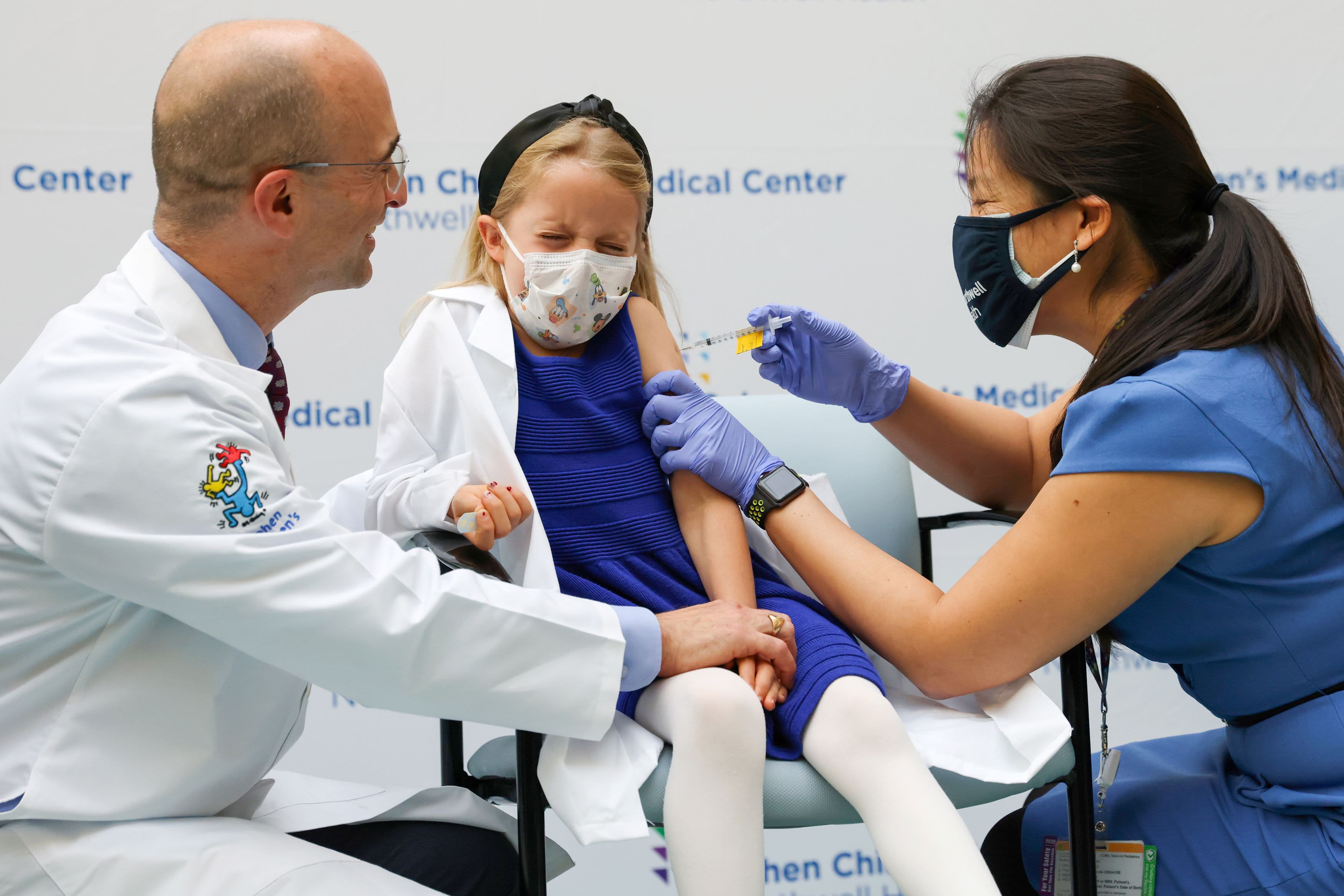
Pfizer Vaccine Was Just 12 Effective For Kids In Omicron Surge Study
How Did The Packaging Industry Deliver The Covid 19 Vaccine Article Packaging Europe
Covid 19 Vaccine Mixing The Good The Bad And The Uncertain
Nine Frequently Asked Questions About Pfizer Covid Pediatric Vaccine Blog Loyola Medicine

Can You Mix Different Covid 19 Vaccines New Research World Economic Forum

The Pfizer Covid 19 Vaccine Info Vegetarian Society

Moderna And Pfizer Covid Vaccines Are Fully Approved By Fda Novant Health Healthy Headlines

Pfizer Seeks Ok Of Updated Bivalent Covid 19 Vaccine Booster For Fall Mint